Substituent dependence of imidazoline derivatives on the capture and release system of carbon dioxide†
Abstract
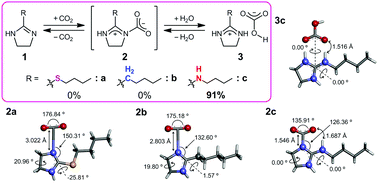
The reversible capture–release behaviors of CO2 by imidazoline derivatives bearing several 2-substituents were investigated under dry conditions containing a very small amount of water. The imidazoline having a cyclic guanidine moiety only captured CO2 quantitatively, and then the cyclic guanidine derivative gave the guanidinium bicarbonate by CO2 fixation together with a slight amount of water. In contrast, the imidazolines bearing n-butylthiol and n-pentyl groups never captured CO2 even with the addition of an equimolar amount of water. Furthermore, the binding structure between CO2 and the cyclic guanidine derivative was studied in detail by elemental analysis, FTIR-ATR, solid-state NMR, TGA, and DFT calculations in order to predict the CO2 capture–release mechanisms depending on the imidazoline structure.


 Please wait while we load your content...
Please wait while we load your content...