Dual-responsive ALS-type organogelators based on azobenzene–cholesteryl conjugates and their self-assemblies†
Abstract
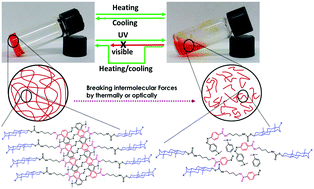
Two structurally isomeric azobenzene- and cholesteryl-based derivatives with varying alkyl chain lengths were developed as ALS-type gelators (N2 and N4) and synthesized and characterized spectroscopically. Of the two, N4 acted as a more efficient gelator than N2 since N4 could gel a larger number of solvents. The critical gelation concentration (CGC) of N4 was found to be less than that of N2 in the same solvent system. The morphological analyses of both gelators using SEM and TEM revealed that N4 exhibited self-assembled fibrous structures, whereas N2 exhibited spherical nanoparticles. The van der Waals interactions between the cholesteryl units, hydrogen bonding between the amide linkages and π–π stacking between the azobenzene units provided the driving force for the aggregation and gel formation. These driving forces were evidenced by temperature dependent 1H-NMR, FTIR and XRD analyses. Increasing the temperature of the gels shifted (upfield and downfield) the protons in the 1H-NMR spectra as well as the absorption bands in the FTIR spectra indicating that the intermolecular forces between the molecules became disrupted and caused gel → sol transitions. These transitions were reversible after cooling to room temperature. Similarly, the gel → sol transitions could be triggered by UV light (due to trans/cis isomerization); however, the transition was irreversible in the presence of visible light due to the formation of the more stable cis isomer. Hence, the gel state could be retained by heating and cooling the cis-conformation. In addition, the length of the molecule as determined by simulation software was found to match the values observed from the XRD analysis, and the interlayer distances were found to be 1.78 and 1.85 nm for N2 and N4, respectively. Based on this evidence, an aggregation mechanism was proposed. The differential scanning calorimetry (DSC) and polarized optical microscopy (POM) results revealed that both gelators exhibited grainy nematic mesophase textures during the heating and cooling cycles. These gelators underwent phase-selective gelation in the solvent mixtures containing gelling and nongelling solvents, which demonstrated the applicability of these gelators for the separation and purification of solvents.


 Please wait while we load your content...
Please wait while we load your content...