A highly elastic and flexible solid-state polymer electrolyte based on ionic liquid-decorated PMMA nanoparticles for lithium batteries†
Abstract
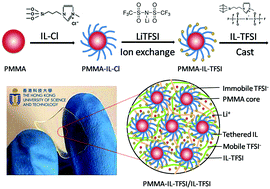
A novel solid-state polymer electrolyte (SPE) based on ionic liquid (IL) and surface functionalized PMMA nanoparticles is reported. It exhibits a much improved ionic conductivity of 5.12 × 10−4 S cm−1 at 30 °C, an enhanced lithium ion transference number, and a wide electrochemical window of up to ∼5.1 V. The enhanced ionic conductivity is attributed to the unique self-assembly of PMMA nanoparticles with surface-tethered IL groups. It is also found that further incorporation of the IL-based phase can significantly increase the ion mobility by providing a microscopic fluidic environment. More interestingly, this SPE exhibits excellent elasticity with an exceptionally high elongation-at-break value of up to 1600%. These attributes enable it to be a high-performance membrane electrolyte and a separator that is suitable for flexible and wearable lithium batteries.


 Please wait while we load your content...
Please wait while we load your content...