Theoretical aspects of the enhancement of metal binding affinity by intramolecular hydrogen bonding and modulating pKa values†
Abstract
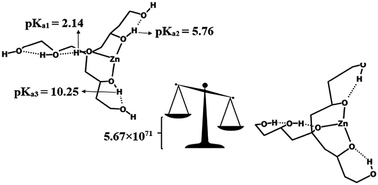
Polyols were used as model ligands for Mg2+, Ca2+, and Zn2+ complexes to study the role of the hydrogen bond network on the metal binding affinity and modulation of successive pKa values using density functional theory. The results confirm that the acidity of polyols dramatically increases upon metal complexation in the order Zn2+ > Mg2+ > Ca2+. For example, the three H-site positions in the hydroxyl groups of the heptaol, bound to Zn2+, are 11.2, 29.9, and 30.9 pKa units (in methanol) more acidic than those of pure heptaol. This acidity enhancement leads to making polyols as good ligands toward complexation. For instance, the formation constants of the heptaol in the presence of Zn2+, Mg2+, and Ca2+ in methanol were 5.67 × 1071, 6.46 × 1066, and 1.26 × 1053 times lower than those in its third deprotonation state, respectively. The natural bond orbital (NBO), quantum theory of atoms in molecules (QTAIM), and reduced density gradient (RDG) analyses show that the intramolecular hydrogen bond network and increment of carbon chain length lead to the enhancement of metal binding affinity. These findings can be used for the manipulation of ligands and metal cations in designing metalloproteins.


 Please wait while we load your content...
Please wait while we load your content...