Electrochemical behavior of glycine-mediated N-doped reduced graphene oxide†
Abstract
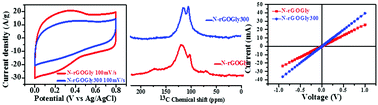
In the present manuscript, N-doped reduced graphene oxide (rGO) has been synthesized by using an environmentally benign amino acid, glycine, as a reducing agent (N-rGOGly) under mild experimental conditions in aqueous medium and has been explored for its electrochemical behavior. It exhibited a fairly high value of specific capacitance (263 F g−1 at 1 A g−1), a high energy density/power density, a high coulombic efficiency, and long cycling stability. Annealing of this sample at a mild temperature of 300 °C produced crystalline N-doped rGO (N-rGOGly300) with a six-fold symmetry having a ‘‘d” spacing of 0.367 nm as was revealed by its HRTEM analysis. XPS, Raman, and 13C NMR analyses exhibited its enhanced graphitic character which was further evidenced by its more than six times increase in conductivity (69.78 S cm−1) as compared to the non-annealed sample (11.4 S cm−1) suggesting its possible applications for the fabrication of electrical devices. Annealing though resulted in the reduction of its pseudocapacitive contribution but it exhibited appreciably higher values of Cs of 191 F g−1 at 1 A g−1 and other electrochemical features.


 Please wait while we load your content...
Please wait while we load your content...