Synthesis, characterization and properties of lanthanide coordination polymers with 3,5-bis(4-carboxyphenylmethyloxy) benzoic acid†
Abstract
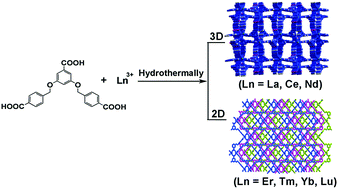
Two types of coordination polymers based on a flexible tripodal multicarboxylate ligand and lanthanide metal ions, namely, [Ln(L)]·4.5H2O (Ln = La (1), Ce (2), Nd (3), named as type I) and [Ln(L)(H2O)2]·3.5H2O (Ln = Er (4), Tm (5), Yb (6), Lu (7), named as type II) (H3L = 3,5-bis(4-carboxyphenylmethyloxy)benzoic acid), have been hydrothermally synthesized and characterized structurally. Single-crystal X-ray diffraction analyses revealed that compounds 1–3 are isostructural, and they crystallize in the orthorhombic Pbcn space group with a 3D framework. In contrast, isomorphic compounds 4–7 exhibit 2D networks in the monoclinic C2/m space group, and were synthesized under experimental conditions similar to 1–3. The structural diversities reveal that the lanthanide contraction has a significant effect on the structural self-assembly process. The PXRD patterns of the hydrated, dehydrated and rehydrated samples of 5 show that the dehydration–rehydration process of 5 is reversible, based on which the alcohol sorption property of 5 was explored. Magnetic susceptibility studies of 2–5 have been carried out, which indicated a antiferromagnetic interaction. The solid-state luminescent behaviors of 2, 3 and 6 were investigated at room temperature. 3 and 6 exhibit the unique emissions of Nd and Yb, respectively.


 Please wait while we load your content...
Please wait while we load your content...