Preparation and characterization of a PFSA–PVDF blend nanofiber membrane and its preliminary application investigation†
Abstract
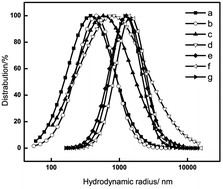
Herein, electrospinnability of perfluorosulfonic acid (PFSA)–polyvinylidene fluoride (PVDF) blends with different ratios of PVDF were investigated in detail. A well-formed nanofiber structure can be obtained only when the minimum ratio of PVDF in the blend reaches 9%. The reason was explained by the change in the properties, such as viscosity, conductivity, and surface tension, and interconnected aggregates in the blend solutions as revealed by dynamic light scattering (DLS) and the viscoelasticity behavior. After this, the property and morphology of the PFSA–PVDF nanofibers were investigated. SEM images showed that the average diameter of the nanofibers ranged from 75 to 170 nm. Small-angle X-ray scattering (SAXS) and DSC results showed that the microstructure of PFSA and the crystallization of PVDF in the nanofiber interfered with each other, which was attributed to the interaction between the backbone of PFSA and PVDF. CA and IEC revealed that the nanofibers were highly hydrophobic. Finally, the PFSA–PVDF nanofiber membranes were evaluated as a catalyst in the esterification reaction and as a separator in Li-ion battery. It was found that PFSA/PVDF nanofiber membrane with high PFSA ratio showed higher capacity and more stable cycle performance after 100 cycles as compared to the PVDF nanofiber, which could be used as a separator in Li-ion battery.


 Please wait while we load your content...
Please wait while we load your content...