Synthesis of 4-aryl-1,2,3-triazolyl appended natural coumarin-related compounds with antiproliferative and radical scavenging activities and intracellular ROS production modification†
Abstract
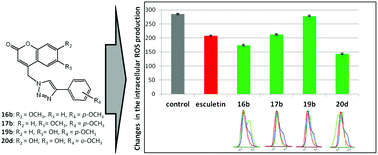
Novel natural coumarin-based umbelliferone, herniarin and esculetin series linked to hydroxy- and methoxy-substituted and non-substituted phenyl rings through a 1,2,3-triazole spacer were provided by environmentally friendly click chemistry under microwave irradiation. The antioxidative activity of the compounds was evaluated by DPPH-scavenging activity and cyclic voltammetry assays. While adjacent 6- and 7-hydroxyl groups in coumarins made a major contribution to antioxidative power and a decrease of ROS production relative to esculetin, a p-methoxy-substituted phenyl moiety had an impact on pronounced and selective antiproliferative activity against chronic leukemia cells in blast crisis (K562) with no significant change in the ROS generation. 6,7-Dihydroxycoumarin can be considered as a promising scaffold with a major contribution to antioxidant potential and, thereby, further structural modification of 6,7-dihydroxycoumarin linked to aryl-1,2,3-triazole may provide a hybrid molecule that may be of interest for potential application to prevent diseases related to the oxidative-stress imbalance.


 Please wait while we load your content...
Please wait while we load your content...