Large magnetic entropy changes in three GdIII coordination polymers containing GdIII chains†
Abstract
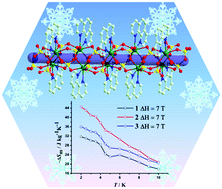
Three chain-based GdIII coordination polymers, namely [Gd2(SO4)3(phen)2(H2O)2]n (1), [Gd2(oda)2(ox)(H2O)6]n (2) and {[Gd2(glu)3(H2O)2]·4H2O}n (3) (phen = 1,10-phenanthroline, H2oda = oxydiacetic acid, H2ox = oxalic acid and H2glu = glutaric acid), have been successfully constructed by using multidentate O ligands under hydro/solvothermal conditions. Complex 1 has a one-dimensional (1D) chain bridged by inorganic sulfate, and complex 2 exhibits a 1D double-chain ladder-shaped structure connected by oxalate. While, complex 3 is a three-dimensional (3D) metal–organic framework composed of 1D chains. All of them display good thermal stabilities and large magnetic entropy changes with −ΔSmaxm > 31 J kg−1 K−1 (7 T), suggesting them to be good candidates for use as cryogenic magnetic refrigerants.


 Please wait while we load your content...
Please wait while we load your content...