Investigation of the protonation state of the macrocyclic {HnP8W48O184} anion by modeling 183W NMR chemical shifts†
Abstract
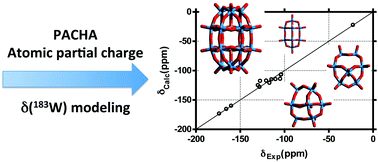
A semi-empirical approach based on the partial charge formalism has been used to calculate 183W NMR chemical shifts in polyoxotungstates (POTs). Good consistency between calculations and experimental measurements allowed determining the main effective parameters for modeling the electronic state of the {WVIO6} centers. As the three 183W NMR chemical shifts of the {P8W48O184} macrocycle exhibit strong pH dependence, partial charge model calculations have been carried out for the investigation of the protonation of the metal–oxo framework. Furthermore, these calculations highlight the effect of the potassium ions embedded within the internal cavity. In this work, NMR parameters were computed using the PACHA software, distinguishing and assigning 183W NMR resonances.


 Please wait while we load your content...
Please wait while we load your content...