Electrodeposition of PANI/MWCNT nanocomposite on stainless steel with enhanced electrocatalytic activity for oxygen reduction reaction and electro-Fenton process
Abstract
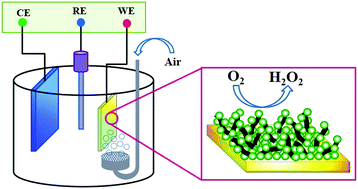
Highly efficient hydrogen peroxide (H2O2) electrogeneration is required in the electro-Fenton (EF) process for the treatment of organic wastewaters. In this study, electrochemical deposition of a polyaniline (PANI)/multi-walled carbon nanotube (MWCNT) nanocomposite was performed to modify stainless steel (SS), as an EF cathode, for the electrogeneration of H2O2. The operating variables, including MWCNT concentration, initial pH and applied potential were optimized using response surface methodology (RSM), regarding the H2O2 concentration as the response. The highest concentration of H2O2 (7.51 mg L−1) was obtained under the following optimal conditions: MWCNT concentration of 2 wt%, initial pH of 2 and applied potential of −0.6 V vs. SCE. The PANI/MWCNT nanocomposite was characterized using Fourier transform infrared spectroscopy (FTIR) and scanning electron microscopy (SEM). Linear scanning voltammetry (LSV) was also used to demonstrate the electrocatalytic activity of the electrodes toward O2 reduction. Finally, the degradation of Rhodamine B (RhB) was evaluated by ultraviolet (UV) spectral analysis and chemical oxygen demand (COD) measurements. Enhanced decolorization of RhB (90%) occurred at the modified cathode, compared to the unmodified steel (61%) after 30 min. The COD removal efficiency reached 55.6% at 3 h, which was more than twice that using the unmodified one (21.4%). Furthermore, the electrochemical energy consumption for COD removal was considerably lower for the modified electrode, than that of the unmodified electrode (4.6 kW h kg−1 COD vs. 22.8 kW h kg−1 COD).


 Please wait while we load your content...
Please wait while we load your content...