A heterogenized chiral imino indanol complex of manganese as an efficient catalyst for aerobic epoxidation of olefins†
Abstract
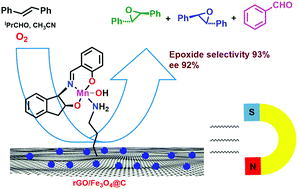
Herein, a new heterogenized chiral catalyst, GFC-[Mn(L)(OH)], was synthesized by grafting the complex [Mn(L)(OH)] on carbon-coated magnetic Fe3O4 nanoparticle-decorated reduced graphene oxide sheets (GFC) through an amine linkage (L = (1R,2S)-1-(N-salicylideneamino)-2-indanol). The catalyst was characterized via FT-IR, UV/vis, XRD, SEM, and vibrating sample magnetometer (VSM) techniques. It exhibited excellent activity and selectivity in the epoxidation of olefins with oxygen in the presence of isobutyraldehyde under mild conditions (conversion 38–98%; selectivity 65–98%; and enantioselectivity 58–100%, except for alpha-methylstyrene). Furthermore, the synergistic effect of the reduced graphene oxide support was observed on the increasing activity, epoxide selectivity, and enantioselectivity. The catalyst can be recovered via magnetic separation from the reaction mixture and recycled five times without any significant loss in its activity. The advantage of this development is the use of both the synergic effect of reduced graphene oxide and the magnetite nanoparticles to obtain an easily recyclable heterogeneous green catalyst. In addition, high asymmetric induction of a rigid indanol-based unit of the ligand results in high enantioselectivity.


 Please wait while we load your content...
Please wait while we load your content...