Synthesis of porous polymeric metalloporphyrins for highly efficient oxidation of cyclohexane in heterogeneous systems†
Abstract
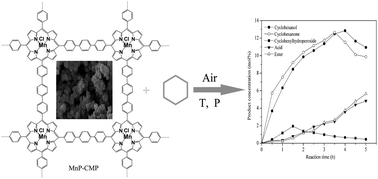
Herein, porphyrin-conjugated organic polymers, Mn(III)P-CMP and Fe(III)P-CMP, were synthesized by Suzuki coupling reaction with manganese/iron tetraphenylporphyrin (T(p-Br)PPMn/FeCl) and 1,4-phenyldiboronic acid as building blocks. The structures of the pore and surface were characterized by nitrogen adsorption and desorption isotherm, FE-SEM, and HR-TEM. The electrochemical behavior of Mn(III)P-CMP was tested by cyclic voltammetry. For the catalytic oxidation of higher inert C–H bond in cyclohexane, Mn(III)P-CMP and Fe(III)P-CMP with hyper-conjugated and microporous structures functioned better than the monomer metalloporphyrin. With MnP-CMP as the preferred catalyst, after 3.5 h of reaction, the yield of cyclohexanol and cyclohexanone was 21.6%, and the conversion of cyclohexane reached 29.9% at the Mn3+/cyclohexane molar ratio of 1/168 000, 150 °C, and 0.8 MPa. Mn(III)P-CMP and Fe(III)P-CMP were structurally stable and insoluble in common solvents and hardly degraded below 300 °C; hence, they had excellent reusability.


 Please wait while we load your content...
Please wait while we load your content...