Increase in chloride retention using anion exchange membranes electrochemically impregnated with polyaniline/sodium polystyrene sulfonate composite deposits
Abstract
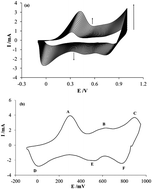
Ion exchange membranes are highly technologically relevant and composite deposits can be used to enhance some of their properties. Because of its exceptional properties, polyaniline (PAni) has been used to house certain species that together confer special characteristics to these membranes. Among these species are polyelectrolytes, which are molecules of high molecular weight that have ionizable groups. In this study, we used sodium polystyrene sulfonate (PSS) incorporated in PAni, which was prepared in two different oxidation states, to selectivity modify the properties of a commercial anion-exchange membrane. These modified membranes were used to selectively separate nitrate ions from a mixture with chloride ions. It was found that the membrane modified with a reduced PAni-composite deposit was more selective toward nitrate ions. This behavior is related to intense repulsive interactions that develop between the composite deposit in the membrane and the anions that try to cross the membrane. In the reduced-composite-modified membrane, the individual effects of the reduced PAni and the PSS combine to reject the chloride ion with greater intensity by virtue of its greater hydration number compared with nitrate, and the hydrophobic characteristics of PAni.


 Please wait while we load your content...
Please wait while we load your content...