A new Schiff-based chemosensor for chromogenic sensing of Cu2+, Co2+ and S2− in aqueous solution: experimental and theoretical studies†
Abstract
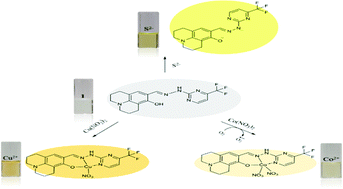
A new Schiff-based multifunctional colorimetric chemosensor 1 was developed for the detection of various analytes (Cu2+, Co2+ and S2−). Sensor 1 could simply monitor Cu2+ and Co2+via the naked eye in an aqueous environment. The quite low detection limits were found to be 0.19 μM for Cu2+ and 0.18 μM for Co2+, which were much lower than the values recommended by the World Health Organization (WHO) for Cu2+ (31.5 μM) and Environmental Protection Agency (EPA) for Co2+ (17 μM). Importantly, 1 showed high preferential selectivity for Cu2+ and Co2+ over competitive metal ions. For practical applications, the sensing abilities of 1 for detecting Cu2+ and Co2+ were examined in real water samples. In addition, 1 exhibited high selectivity for S2− even in the presence of other anions without any interference. Moreover, the sensing mechanisms of 1 toward Cu2+, Co2+ and S2− were explained by theoretical calculations.


 Please wait while we load your content...
Please wait while we load your content...