Different pH triggered aggregate morphologies in sodium oleate–cationic surfactants mixed systems†
Abstract
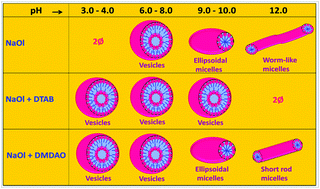
The aggregate morphologies in aqueous solutions of sodium oleate (NaOl) + dodecyldimethylamine oxide (DMDAO, zwitterionic) or dodecyltrimethylammonium bromide (DTAB, cationic) were investigated over a wide pH range (3 to 13) by tensiometry, rheology, scattering and spectral measurements. NaOl and DMDAO show interesting micellar behavior with pH changes. NaOl forms worm-like micelles at higher pH (∼12) and ellipsoidal micelles in the pH range ca. 9–11, which transform into vesicles at pH ca. 7 to 8. Due to hydrolysis, NaOl cannot be used in acidic pH, but this deficiency can be conquered by the mixed system of NaOl with DTAB and DMDAO. NaOl strongly interacts with DTAB to form vesicles in a wide pH range. The DMDAO/NaOl mixed system forms ellipsoidal micelles on mixing, which turn into vesicles at pH below 9 and short rods at higher alkaline pH without undergoing any precipitation. The effect of salt on the transition of NaOl as well as mixed micelles has also been described. These studies provide an alternative to the restriction of the use of fatty acid soaps in acidic pH, and are worthwhile for industrial and domestic purposes.


 Please wait while we load your content...
Please wait while we load your content...