Substituted bidentate and ancillary ligands modulate the bioimaging properties of the classical Re(i) tricarbonyl core with yeasts and bacteria†
Abstract
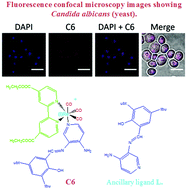
Rhenium(I) tricarbonyl complexes with heteroaromatic ligands have been intensely investigated with respect to their properties as imaging probes, although they have only recently been tested in vivo. In this context, fac-Re(CO)3(N,N)L complexes (N,N: substituted bidentate ligand; L: ancillary ligand) are the most studied complexes due to their photophysical properties. However, the role of the N,N bidentate ligand in classical fac-Re(CO)3(N,N)L complexes (i.e. where L is a halogen such as Br) has not been explored regarding cytotoxicity and staining capabilities in walled cells (i.e. yeasts and bacteria). In the present study, we tested different rhenium(I) tricarbonyl complexes of type fac-Re(CO)3(N,N)Br [where N,N are 1,10-phenanthroline (phen) (C1); 5,6-dione-1,10-phenanthroline (dione) (C2); 2,2′-bpy (bpy) (C3); 4,4′-dimethyl-2,2′-bpy (dmb) (C4); and 4,4′-diethanoate-2,2′-bpy (deeb) (C5)] in order to characterize the properties of the N,N bidentate ligand in cellular biomarkers. We also compared these classical rhenium(I) tricarbonyl complexes (C1 to C5) with a fac-Re(CO)3(deeb)L+ complex, where L is the Schiff base (E)-2-((3-amino-pyridin-4-ylimino)-methyl)-4,6-di-tert-butylphenol, with respect to its potential for cell labelling. In our study, we found that both the N,N substituted bidentate ligand and the ancillary ligand L contributed to modulating the suitability in cell bioimaging, showing that it is possible to perform molecular engineering design to obtain improved biomarkers for walled cells, and eventually for other cell types.


 Please wait while we load your content...
Please wait while we load your content...