An efficient and mild oxidative amidation of aldehydes using B(C6F5)3 as a catalyst and biological evaluation of the products as potential antimicrobial agents†
Abstract
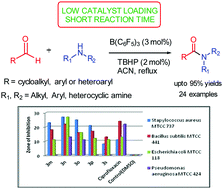
A mild and efficient protocol for oxidative amidation of diverse aldehydes with amines was developed using 3 mol% tris(pentafluorophenyl)borane and tert-butyl hydroperoxide to generate the corresponding amides in good to excellent yields. This method has significant advantages such as short reaction time, low toxicity, low catalyst loading, and being environmentally friendly and an operationally simple procedure. Acid labile protecting groups such as acyl and Boc displayed tolerance under the present catalytic system. Applicability in large scale synthesis of amides is an added advantage of the protocol. Moreover, direct amidation of aldehydes using substituted N-benzylanilines for the synthesis of corresponding amides was devised using the present catalytic system. Compounds 3n and 3o displayed promising antimicrobial activity against Staphylococcus aureus (Gram positive) with MIC ranging from 0.4–0.7 μg mL−1 and against Escherichia coli (Gram negative) with MIC 0.7–1.2 μg mL−1 with reference to the standard drug Ciprofloxacin.


 Please wait while we load your content...
Please wait while we load your content...