Glycerol electro-oxidation in alkaline media using Pt and Pd catalysts electrodeposited on three-dimensional porous carbon electrodes
Abstract
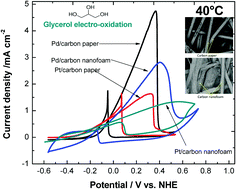
In this work, Pd and Pt electrocatalysts were electrodeposited on three-dimensional carbon paper and carbon nanofoam with the purpose of increasing the catalytic area to improve the glycerol electro-oxidation. SEM and cross-sectional SEM micrographs showed that Pd and Pt particles were well-distributed over the entire three-dimensional electrode surfaces. Commercial Pd/C and Pt/C catalysts deposited by the spray method were used for comparison, showing lower surface area (SA) utilization than those electrodeposited. The electrodeposition effectiveness to cover the electrode surfaces was evaluated by changes in overall SA and through the calculation of electrochemically active surface area (EASA) and specific surface area (SSA). Despite the larger EASA values found for Pd and Pt on nanofoam, Pt on paper showed the highest utilization of the surface area, obtaining an SSA of 58.1 m2 g−1. Moreover, the electrodeposition of Pd and Pt dramatically increased the EASA versus the geometrical area, improving this ratio 16 (Pd on paper), 151 (Pt on paper), 158 (Pd on nanofoam) and 277-fold (Pt on nanofoam). The electrodeposited porous Pt electrodes showed good activity for glycerol oxidation, exhibiting a more negative potential than Pd-based materials. However, for fuel cell applications operated at intermediate temperatures, Pd on carbon paper is the optimal candidate to be used as an anode because of its high current density and excellent poisoning tolerance.


 Please wait while we load your content...
Please wait while we load your content...