Synthesis and biological evaluation of new fluconazole β-lactam conjugates linked via 1,2,3-triazole†
Abstract
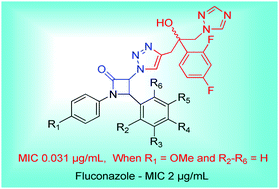
Novel 1,2,3-triazole-linked β-lactam–fluconazole conjugates 12(a–l) were designed and synthesized. The compounds showed potent antifungal activity against two pathogenic Candida strains; Candida albicans ATCC 24433 and Candida albicans ATCC 10231 with MIC values in the range of 0.0625–2 μg mL−1. Compounds 12h, 12j and 12k showed promising antifungal activity against all the tested fungal pathogens except C. neoformans ATCC 34554 compared to fluconazole. Compound 12j in which the β-lactam ring was formed using para-anisidine and benzaldehyde was found to be more potent than fluconazole against all the fungal strains with an IC50 value of <0.015 μg mL−1 for Candida albicans (ATCC 24433). Mechanistic studies for active compounds revealed that the antifungal action was due to ergosterol inhibition. Compounds 12h and 12j at a concentration of 0.125 μg mL−1 caused 91.5 and 96.8% ergosterol depletion, respectively, compared to fluconazole which at the same concentration caused 49% ergosterol depletion. The molecular docking study revealed that all the fluconazole β-lactam conjugates 12(a–l) could snugly fit into the active site of lanosterol 14α-demethylase (CYP51) with varying degrees of affinities. As anticipated, the binding energy for compound 12j (−58.961 kcal mol−1) was much smaller than that for fluconazole (−52.92 kcal mol−1). The synthesized compounds have therapeutic potential for the control of candidemia.


 Please wait while we load your content...
Please wait while we load your content...