Use of apomyoglobin to gently remove heme from a H2O2-dependent cytochrome P450 and allow its reconstitution†
Abstract
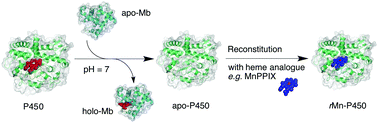
The heme of hydrogen peroxide-dependent cytochrome P450BSβ (P450BSβ) was removed by apomyoglobin under mild conditions to give apo-P450BSβ without the need for acidic conditions and organic solvents. The circular dichroism spectrum of the apo-P450BSβ was essentially identical to that of holo-P450BSβ, showing a small structural change resulting from the removal of heme using apomyoglobin. The apo-P450BSβ was reconstituted with hemin or manganese protoporphyrin IX (MnPPIX), and the resulting reconstituted P450BSβ catalyzed the one-electron oxidation of guaiacol using hydrogen peroxide as an oxidant. A higher catalytic activity was observed for P450BSβ reconstituted with MnPPIX when meta-chloroperoxybenzoic acid (mCPBA) was used as the oxidant.

 Please wait while we load your content...
Please wait while we load your content...