Modulated emission from dark triplet excitons in aza-acene compounds: fluorescence versus phosphorescence†
Abstract
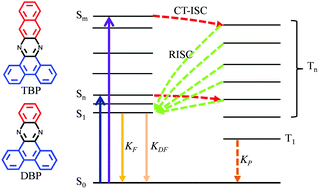
In the field of organic light-emitting diodes (OLEDs), research interests focus on making the optically dark triplet excitons shine in order to increase the electro-optic conversion efficiency of devices. In this work, two kinds of phenazine compounds, i.e. dibenzo[a,c]phenazine (DBP) and tribenzo[a,c,i]phenazine (TBP), were synthesized and used as model compounds to regulate the emission efficiency of the dark triplet excitons by chemical modification. Charge-transfer induced ultrafast intersystem crossing (CT-ISC) with a time constant of ∼1 ps was observed for these two phenazine derivatives upon photoexcitation with a high triplet yield of 77.1% for DBP and 58.7% for TBP. The triplet excited states of DBP can produce ultra-long phosphorescence with lifetime as long as 318 ms at 77 K. The quantum yield for phosphorescence (ΦP) is determined to be 8.45%. In sharp contrast, the triplet-excited 3TBP* undergoes an efficient reverse intersystem crossing (RISC) process, resulting in bright delayed fluorescence emission with negligible phosphorescence. A controllable luminescence behavior from the triplet states between fluorescence and phosphorescence in phenazine derivatives is demonstrated. Theoretical calculations reveal that the structure-dependent triplet evolution is due to the charge-transfer induced energy level alignment within these compounds. Our results may have potential applications in the design of OLEDs and high triplet yield pure organic materials.


 Please wait while we load your content...
Please wait while we load your content...