Ambipolar azomethines as potential cathodic color switching materials†
Abstract
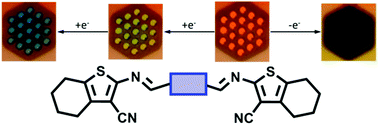
A series of conjugated azomethine triads were prepared from a 2-amino-3-nitrilethiophene derivative (5) and four different aromatic dialdehydes. The conjugated azomethines were targeted to investigate the effect of the different aromatics on the spectroscopic, electrochemical, and spectroelectrochemical properties. It was found that the triads could be both electrochemically oxidized and reduced, with the reversibility of the process and the redox potential being contingent on the central aromatic. The triads also had strong absorptions in the visible and the electrochemically produced states were red-shifted by more than 200 nm relative to their original neutral state. When the central aromatic was a triphenylamine, reversible color changes were observed upon electrochemical oxidation/neutralization. The resulting oxidized state absorbed more than 350 nm across the visible spectrum. The perceived color change upon electrochemical oxidation was from orange to black. Similarly, reversible color changes were observed upon electrochemical reduction/neutralization when the central aromatic was a phenyl, thiophene, and EDOT.


 Please wait while we load your content...
Please wait while we load your content...