Hypervalent iodine catalysis for selective oxidation of Baylis–Hillman adducts via in situ generation of o-iodoxybenzoic acid (IBX) from 2-iodosobenzoic acid (IBA) in the presence of oxone†
Abstract
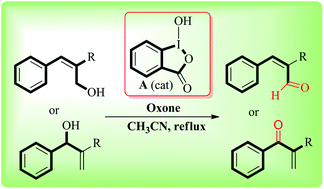
An efficient, environmentally benign, eco-friendly protocol for selective oxidation of primary and secondary Baylis–Hillman alcohols to the corresponding carbonyl compounds has been developed. We have demonstrated the catalytic use of o-iodoxybenzoic acid (IBX) generated in situ from 2-iodosobenzoic acid (IBA) in the presence of oxone (2KHSO5·KHSO4·K2SO4) as a co-oxidant. This efficient method notably enables better to high yields without the use of any toxic heavy metals and the direct use of tricky IBX. Furthermore, the synthesized catalyst could be recovered conveniently using a reductive work-up.

 Please wait while we load your content...
Please wait while we load your content...