New relationship models for solvent–pyrene solubility based on molecular structure and empirical properties†
Abstract
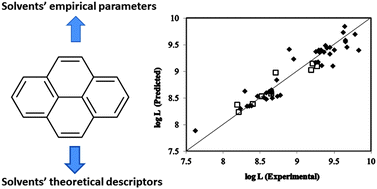
Pyrene is a fluorescent probe and a well-known compound in dye, plastic and petrochemical industries and thus the solubility of pyrene in different solvents is very important. A three parametric LSER model based on empirical scales and a five parametric linear QSPR model containing theoretical descriptors are suggested to correlate the Ostwald solubility coefficient of pyrene with structural/empirical properties of various organic solvents. The proposed QSPR and LSER models covered 85% and 88% of data variance respectively and both models were validated using different statistical approaches such as cross-validation and external test sets. The models not only showed a good internal prediction ability, but were also capable of predicting the solubility of pyrene in external test solvents that were not utilized in the model construction step. The advantages and limitations of the two applied approaches were discussed. It was revealed that in addition to structural parameters like electrotopological state and mean atomic composition, some empirical features such as the basicity of solvents and their molar transition energy have significant roles in the solubility of pyrene.


 Please wait while we load your content...
Please wait while we load your content...