Selectivity and activity in catalytic hydrogenation of azido groups over Pd nanoparticles on aluminum oxy-hydroxide†
Abstract
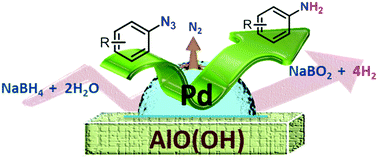
Azidoarenes involving various functional groups were successfully reduced to aniline derivatives using commercially available aluminium oxy-hydroxide-supported palladium (Pd/AlO(OH)) nanoparticles (0.5 wt% Pd) in an aqueous medium with sodium borohydride as the hydrogen source. To develop the green process, water was utilized in conjunction with methanol. The results demonstrated that the halogen substituted azidoarenes were selectively converted to the corresponding aniline compounds without dehalogenation. In general, all of the reactions were completed within 10–30 min at room temperature with yields of over 99%. In order to optimize the reaction conditions, the parametric effects of the solvent type and the amount of the catalyst/NaBH4 were examined. Consequently, for the first time, a novel, practical and environmentally friendly process was developed for the conversion of azidoarenes to aniline derivatives in the fluence of Pd/AlO(OH) nanoparticles.

 Please wait while we load your content...
Please wait while we load your content...