A new series of bipyridine based chiral organocatalysts for enantioselective Henry reaction†
Abstract
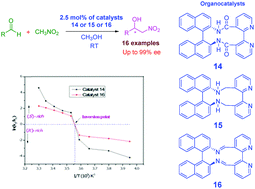
A series of binaphthol based chiral organocatalysts were synthesized and applied as metal-free organocatalysts in the enantioselective Henry reaction. These organocatalysts enabled the Henry reaction with a lower concentration of catalysts at room temperature affording the desired S- or undesired R-enantiomers. The formation of R- and S-enantiomers of β-nitroalcohol products strongly depends on the temperature/substrate inversion of configuration for the effective catalytic enantioselective Henry reaction in high yields (up to 97%) with excellent enantioselectivities (up to 99% ee).

 Please wait while we load your content...
Please wait while we load your content...