A novel approach for the synthesis of imidazo and triazolopyridines from dithioesters†
Abstract
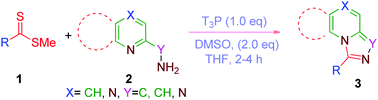
T3P–DMSO mediated desulfurative cyclization of in situ generated thioamides serves as an efficient and versatile method for the synthesis of imidazo[1,5-a]pyridines and [1,2,4]-triazolo[4,3-a]pyridines with good to excellent yields. Substrates such as 2-methylaminoquinoline and pyrazin-2-yl-methanamine also undergo the corresponding reactions at room temperature. This efficient protocol has several advantages such as mild conditions, short reaction time, operational simplicity and high yields.

 Please wait while we load your content...
Please wait while we load your content...