Synthesis, characterization and biological activities of 3-aryl-1,4-naphthoquinones – green palladium-catalysed Suzuki cross coupling†
Abstract
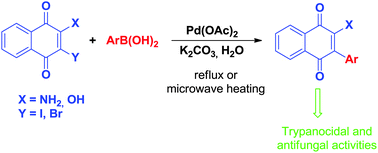
Quinones are important scaffolds that are present in a variety of natural products or synthetic bioactive molecules. Arylation is an important strategy for accomplishing structural modifications, leading to new potential candidates for use as drugs. In the present work, palladium-catalysed, ligandless and phosphine-free Suzuki coupling reactions between 2-hydroxy-3-iodo-1,4-naphthoquinone and boronic acids were employed to prepare several 2-hydroxy-3-aryl-1,4-naphthoquinones in aqueous conditions using microwave irradiation or conventional heating. Because of the biological activities of quinones, which are related to their ability to accept electrons to form semiquinones and hydroquinones, the electrochemical behaviour of the synthesized molecules was investigated. The Osiris and Molinspiration Cheminformatics programs, utilizing in silico analyses, imply that these naphthoquinones are candidates for use as drugs which was reinforced by the outcomes of the in vitro antifungal and trypanocidal activity tests. Our in vitro data indicated a MIC value of 8 μg mL−1 against Candida albicans ATCC 24433 strains, and an EC50 of 0.67 μM with respect to trypanocidal activity against Trypanosoma cruzi epimastigote strains (Y).


 Please wait while we load your content...
Please wait while we load your content...