Mechanistic study on the facet etching effect of silver nanoprisms in the presence of halide ions and their application in the colorimetric sensing of metformin hydrochloride†
Abstract
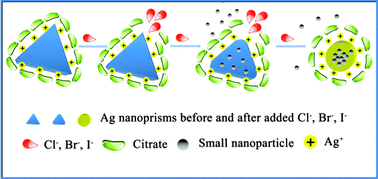
The effects of halide ions on the morphology of Ag nanoprisms are presented herein through the use of UV-Vis spectroscopy, transmission electron microscopy and X-ray photoelectron spectroscopy. F− has no effect on the UV-Vis spectrum of Ag nanoprisms; however, when Cl−, Br− or I− ions are added to the Ag nanoprisms solution, these ions combine with the facet [110] surface, which has the highest crystal surface energy and is not completely protected, and form a precipitant with Ag+. Thus, small nanoparticles of Ag atoms are etched from the vertex areas of the Ag nanoprisms, making the Ag nanoprisms gradually turn into nanodisks. The lowest concentration order of halide ions that can etch Ag nanoprisms is I− < Br− < Cl−, which is consistent with the Ksp order. To examine the feasibility of this approach, we selected Ag nanoprisms as a visual colorimetric probe for metformin hydrochloride (metformin HCl). A pH value of 6.94, VAgNO3 of 0.33 mL and T of 293 K were selected as the optimum values according to response surface methodology (RSM). We could detect the metformin HCl by the naked eye according to the colour change or by a monitor following the spectral changes of A692.

 Please wait while we load your content...
Please wait while we load your content...