Metal-free catalytic synthesis of diaryl thioethers under mild conditions†
Abstract
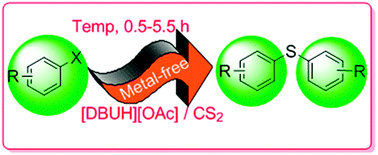
A novel method to synthesize diaryl thioethers from aryl halides and carbon disulfide catalyzed by the 1,8-diazabicyclo[5,4,0]undec-7-enium acetate [DBUH][OAc] ionic liquid (IL) under solvent-free conditions has been developed. This metal-free catalytic system displayed high efficiency for coupling aryl halides with CS2 to deliver thioethers. Compared to the conventional methods, no metal catalyst was needed, instead of which the DBU-based ILs played the catalyst and solvent roles simultaneously. Some reactions were carried out under mild conditions (55 °C, 0.5 h), giving moderate to high yields. Moreover, compared with the rare reports, the reaction of the aryl chlorides and fluorides with CS2 could smoothly react in this catalytic system. The products were easily separated from the ILs which could be reused at least three times. The present method provides an efficient and environment-friendly catalytic approach to synthesize diaryl thioethers.

 Please wait while we load your content...
Please wait while we load your content...