Dinuclear cobalt(iii) and mixed valence trinuclear MnIII–MnII–MnIII complexes with a tripodal bridging pyridylaminophenol ligand†
Abstract
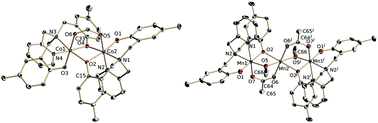
A dinuclear cobalt(II), [Co2(κ4-O,O′,N,N′-L)2(μ-O,O′-HCOO)](ClO4)·(C2H5)2O (1) and a linear mixed valence trinuclear manganese, [Mn3(κ4-O,O′,N,N′-L)2(μ-CH3O)2(μ-O,O′-CH3COO)2]·2(C2H5)2O (2) complexes with the ligand N-(2-pyridyl-methyl)-N,N-bis-[2′-hydroxy-5′-methyl-benzyl]-amine (H2L) are reported. For both complexes the ligand is present in the deprotonated form. The coordination sphere of the cobalt centres can be described as slightly distorted octahedral with two bridging O-phenoxo, one N-pyridine, one N-amine, one terminal O-phenoxo and one bridging O-formate donor atoms. Interestingly, the bridging formate resulted from the aerial oxidation of methanol. The manganese complex (2) has a linear mixed valence MnIII–MnII–MnIII, with the MnII and MnIII centres bridged by alkoxo, carboxylate and phenoxo groups. Cyclic voltammetry studies show both metal and ligand centred redox processes consistent with the structure of the complexes. Complex (2) has an S = 3/2 ground state; magnetic susceptibility measurements indicate a weak antiferromagnetic interaction. The best fit of the magnetic susceptibility data as a function of temperature was obtained using a conventional trinuclear linear model [H = −2J(S1S2 + S1S3)] with J = −0.79 cm−1 and g = 1.99.

 Please wait while we load your content...
Please wait while we load your content...