Topological and packing mode modification for solid-state emission enhancement of bis(perfluorostyryl)furan derivatives†
Abstract
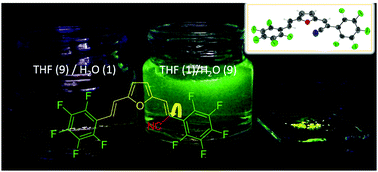
An unsymmetric bis(perfluorostyryl)furan with a cyanovinyl unit was prepared (F1). Its crystallographic structure was resolved along with its cyano vinylene (1) and unsubstituted (2) aldehyde precursors. F1 was found to form ribbons involving C–H⋯F interactions, while also having multiple intermolecular contacts, including C–F⋯Fπ and π–π interactions. These contacts also occurred when F1 aggregated in 9 : 1 water/THF mixtures. When the supramolecular contacts are engaged, the emission intensity of F1 increases, with absolute emission yields of 9 and 25% occurring in aggregates and powder, respectively.

 Please wait while we load your content...
Please wait while we load your content...