Effects of anion substitution on hydration, ionic conductivity and mechanical properties of anion-exchange membranes
Abstract
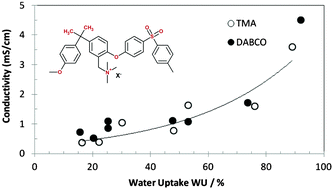
Anion-conducting ionomers were synthesized by the chloromethylation of polysulfone (PSU) followed by the formation of quaternary ammonium groups by a reaction with trimethylamine (TMA) or 1,4-diazabicyclo[2.2.2]octane (DABCO). The degree of functionalization was determined by 1H NMR and titration. Anions (F−, Cl−, Br−, SO42−, NO3−, CO32−, HCO3−, CH3CO2−, and OH−) were substituted by ion exchange in aqueous solution. Water uptake, ionic conductivity and mechanical properties of various ionomers were determined. Hydration has a large influence on both ionic conductivity and mechanical properties: ionic conductivity increases with water uptake, whereas the Young’s modulus decreases. Hydroxide and fluoride containing ionomers present a particularly large ionic conductivity.

 Please wait while we load your content...
Please wait while we load your content...