Synthesis and structural chemistry of bicyclic hexaaza-dithia macrocycles containing pendant donor groups†
Abstract
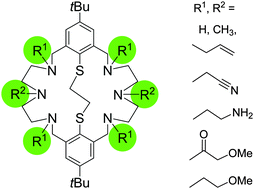
A short and efficient synthesis of a series of macrobicyclic aza-thioethers with pendant allyl (8, 13, 14), cyanethyl (15), 3-aminopropyl (16), 2-methoxyacetyl (17, 19), 2-methoxyethyl (18, 20), and tert-butyloxycarbonyl substituents (22, 23) has been achieved. The parent macrobicycles 1 and 2 are readily alkylated without overalkylation and without affecting the masked thiolate functions. The protocol is also feasible for the synthesis of macrobicycles with different alkyl groups on the benzylic and central nitrogen atoms of the linking diethylene triamine units. The identity of the compounds was substantiated using ESI MS, FT-IR, 1H-NMR, and 13C-NMR spectroscopy. The crystal and molecular structures of six compounds (8, 15, 17·3DMSO, 19·2DMSO·2H2O, 20 and 23) were additionally solved. The macrocycles are rather flexible and can adopt folded or stepped conformations. The ability of the compounds to form inclusion complexes with DMSO is also demonstrated. The crystal structures are governed by extensive inter- and intramolecular CH⋯π interactions.
- This article is part of the themed collection: Nitrogen Ligands

 Please wait while we load your content...
Please wait while we load your content...