Cesium salts of niobo-tungstate isopolyanions with intermediate group V–group VI character†
Abstract
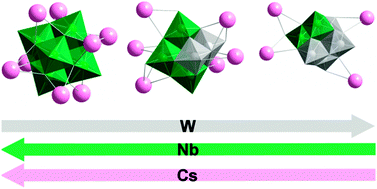
Alkali metal salts of polyoxometalates (POMs) of the group VI elements (W and Mo) and polycoltanates (Nb and Ta POMs) exhibit opposing trends in their solubility in water and ion-association in solution. Mixed clusters of these two group V metals and tungsten provide an opportunity to probe the reversal in these trends and to understand their origin. A review of a classic study of mixed Nb/W clusters and our own work in Ta/W polyanions have led us to isolate Cs+/Na+ salt of [Nb4W2O19]6− and two salts, Cs+/Na+ and pure Cs+, of [Nb2W4O19]4− by using peroxoniobate ([Nb(O2)4]3−) instead of hexaniobate ([Nb6O19]8−) as the niobium source. Crystallographic analysis shows that Cs+-bonding to clusters increases with Nb-content, following the trend observed in our previous studies of hexaniobate in solution. Fragmentation by ESI-MS suggests that niobium-rich [Nb4W2O19]6− is less stable than isostructural [Nb2W4O19]4− and this technique, together with FTIR, confirms the predominance of the cis-isomer in the cluster structures. The mixed-metal composition of these isopolyanions is reflected in the crystallographic bond lengths and in the positions of the absorption bands in the UV spectra. DFT calculations reveal that the HOMO–LUMO energy gap widens with increasing Nb content in the cluster framework – an effect ascribed to the overall poorer mixing of Nb4d, versus W5d, atomic orbitals with the corresponding O2p orbitals.
- This article is part of the themed collection: Emergent Polyoxometalates and Soft-oxometalates

 Please wait while we load your content...
Please wait while we load your content...