Synthesis of ester-substituted dihydroacridine derivatives and their spectroscopic properties†
Abstract
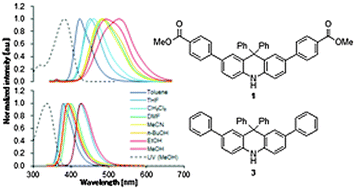
Three dihydroacridine derivatives, 2,7-bis(4-methoxycarbonylphenyl)-9,9-diphenyl-9,10-dihydroacridine (1), 2,8-bis(4-methoxycarbonylphenyl)-10,10-diphenyl-5,10-dihydrophenazasiline (2), and 2,7,9,9-tetraphenyl-9,10-dihydroacridine (3), were prepared and their spectroscopic properties were investigated. These compounds exhibited relatively high quantum yields in a range of solvents. The emission spectra of 1 and 2 displayed large solvatochromic shifts, while the fluorescence solvatochromic behavior was not observed in 3. The intramolecular charge transfer (CT) process from the electron donating moiety at the NH site to the electron withdrawing ester moiety occurs in the excited states of 1 and 2. The increase in the dipole moment induced by the CT process was determined to cause the positive fluorescence solvatochromism. The differences between the excited and ground state dipole moments based on the Lippert–Mataga expression were estimated. The effect of the push–pull substitution in the dihydroacridine π-conjugated system was also discussed using a computational method.

 Please wait while we load your content...
Please wait while we load your content...