A theoretical insight into atmospheric chemistry of HFE-7100 and perfluoro-butyl formate: reactions with OH radicals and Cl atoms and the fate of alkoxy radicals†
Abstract
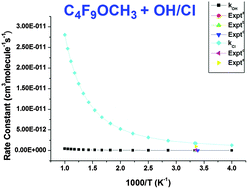
A mechanistic study on the hydrogen abstraction reaction of HFE-7100 (n-C4F9OCH3) with atmospheric oxidants has been carried out in the gas-phase by performing DFT-based M06-2X/6-31+G(d,p) calculations. We observe a complex mechanism due to the presence of the pre-reactive and post-reactive complexes at the entrance and exit channels, respectively. The standard enthalpies of formation for species and bond dissociation energy for C–H bonds are also reported. The rate expressions were determined for reaction channels in the broad temperature range of 250–1000 K. The rate coefficients over the studied temperature range yield the following Arrhenius expressions (cm3 molecule−1 s−1): kOH = 2.87 × 10−24·T3.66 exp(527/T) and kCl = 1.68 × 10−19·T2.71 exp(207/T). At 298 K, the results were found to agree comparatively well with modest differences in rates for reactions. Finally, the atmospheric lifetime and global warming potential of HFE-7100 are computed to be 2.12 years and 155.3, respectively. We also investigated the reactivity of the alkoxy radical (C4F9OCH2O˙), considering three decomposition channels including oxidative pathways. Based on thermochemical data, we can speculate that oxidative pathways are dominant for the decomposition of C4F9OCH2O˙ radicals, which is in concurrence with experimental observations. The radiative efficiency and global warming potential of fluoroesters (C4F9OC(O)H) are also calculated and compared with parent species.

 Please wait while we load your content...
Please wait while we load your content...