Molecular modeling and synthesis of some new 2-imino-4-thiazolidinone derivatives with promising TNF-α inhibitory activity†
Abstract
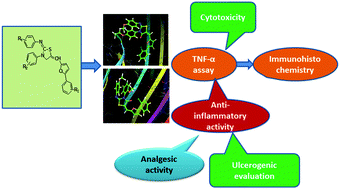
A new series of thirty two 2-imino-4-thiazolidinone derivatives were synthesized, and the synthesized compounds were docked for in silico studies against the TNF-α target. The predicted results were confirmed through an in vitro TNF-α study which revealed that compounds 3f and 3g showed better TNF-α inhibition as compared to the standard drug indomethacin without causing any cytotoxicity. Fourteen compounds exhibiting significant in vitro TNF-α activity were further tested for in vivo anti-inflammatory activity by a carrageenan induced method. Compounds 3f and 3g showed better inhibition of inflammation in vivo as compared to the standard drug without causing any damage to the stomach. Furthermore, an immunohistochemical study showed that the compounds 3f and 3g exhibited better reduction in protein expression of TNF-α as compared to indomethacin. The in silico, in vitro and in vivo studies suggested that the compounds 3f and 3g might be considered as potent anti-inflammatory agents.

 Please wait while we load your content...
Please wait while we load your content...