Novel 1,3,4-oxadiazole motifs bearing a quinoline nucleus: synthesis, characterization and biological evaluation of their antimicrobial, antitubercular, antimalarial and cytotoxic activities†
Abstract
A series of quinoline based 1,3,4-oxadiazole derivatives (8a–l) were synthesized by a chloro-amine coupling reaction approach with different catalysts and solvents. Substituted 1,3,4-oxadiazole intermediates 7a–c were obtained from 2-substituted-N-phenylhydrazinecarbothioamides 6a–c by cyclization with different cyclizing reagents such as mercuric acetate, lead dioxide, iodobenzenediacetate (IBD) and aqueous sodium hydroxide with iodine in aqueous potassium iodide to identify the most effective reaction conditions, in which iodobenzenediacetate was found to be an extremely good catalyst. The structures of the title compounds were confirmed by FT-IR, 1H NMR, 13C NMR and mass spectrometry. The synthesized molecules were evaluated for their antibacterial, antifungal, antituberculosis and antimalarial activities. A brine shrimp bioassay was carried out to study the in vivo cytotoxic properties of the most highly active compounds of the in vitro biological evaluation.
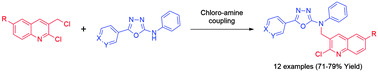

 Please wait while we load your content...
Please wait while we load your content...