Synthesis, structures, and properties of two magnesium silicate fluorides Mg5(SiO4)2F2 and Mg3SiO4F2†
Abstract
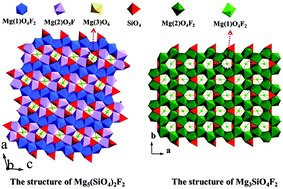
Two magnesium silicate fluorides, Mg5(SiO4)2F2 (compound 1) and Mg3SiO4F2 (compound 2), have been successfully synthesized by solid-state reactions for the first time. Compound 1 crystallizes in the space group P21/c (no. 14) of the monoclinic system, whereas the latter belongs to the space group Pnma (no. 62) of the orthorhombic system. As for the crystal structure, compound 1 can be described on the basis of a “framework” made by Mg1O4F2 and Mg2O5F octahedra forming many rings, in which the Mg3 and Si atoms reside. Compound 2 can also be described as the rings created by Mg2O4F2 octahedra, in which the Mg1 and Si atoms reside. Thermal analysis and the infrared spectrum of compound 1 were measured. In addition, the UV-Vis-NIR diffuse-reflectance spectrum exhibits that the relatively wide band gap is about 5.32 eV for compound 1, which is consistent with the theoretical value. The band structure and linear optical properties of title compounds are also theoretically calculated.

 Please wait while we load your content...
Please wait while we load your content...