Acidity of the methyne group of poly(4-vinylpyridine) leads to side-chain protonation in pyridine†
Abstract
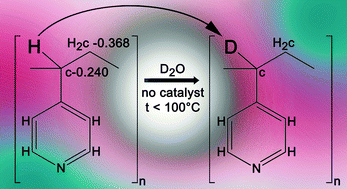
Poly(4-vinylpyridine) swollen in pyridine displays changes in electrical conductivity in response to white light and to low level thermal perturbation; protonation of the side-chain nitrogen is believed to play a role. Here we present spectroscopic evidence that the proton donor is the methyne group CH on the polymer chain.

 Please wait while we load your content...
Please wait while we load your content...