Synthesis and optical and electrochemical properties of polycyclic aromatic compounds with S,S-dioxide benzothiophene fused seven rings†
Abstract
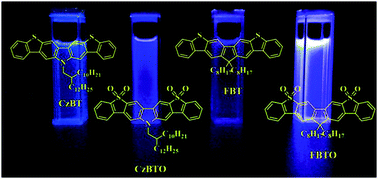
A series of novel, linearly fused polycyclic aromatic compounds, N-(2-decyltetradecyl)carbazole bis[2,3-b;6,7-b]benzo[d]thiophene-S,S-dioxide (CzBTO) and 9,9-dioctylfluorene bis[2,3-b;6,7-b]benzo[d]thiophene-S,S-dioxide (FBTO), incorporating the S,S-dioxide benzothiophene unit, were efficiently synthesized and characterized in detail. The key step in preparing CzBTO and FBTO was related to the intramolecular electrophilic cyclizing reaction induced by triflic acid, in which the corresponding methyl sulfoxides in the activated aromatic building blocks enabled regioselective ring cyclization. CzBTO and FBTO showed very deep blue emission in the photoluminescence spectra, with emission peaks at 426 and 407 nm, respectively. FBTO had a high photoluminescence quantum yield of 77% in tetrahydrofuran solution. Density functional theory and electrochemical studies showed that the CzBTO and FBTO had large band gaps and very low highest occupied molecular orbital energy levels, which indicated that these two compounds had both high thermal and optical stability. From the electrochemical measurements, the highest occupied molecular orbital energy levels were −6.04 and −6.20 and the lowest unoccupied molecular orbital energy levels were −2.74 and −2.80 eV, with band gaps of 3.3 and 3.4 eV for CzBTO and FBTO, respectively.

 Please wait while we load your content...
Please wait while we load your content...