The solvatochromism and aggregation-induced enhanced emission based on triphenylamine-propenone†
Abstract
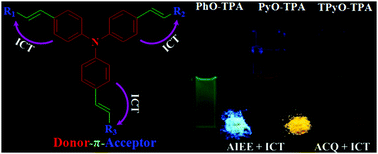
Three new donor–π bridge–acceptor (D–π–A) compounds based on triphenylamine-propenone, namely 4-(1-phenylprop-2-en-1-one-3-yl)triphenylamine (PhO-TPA), 4-(1-(pyridin-2-yl)prop-2-en-1-one-3-yl)triphenylamine (PyO-TPA) and 4,4′,4′′-(tri(1-(pyridin-2-yl)prop-2-en-1-one-3-yl))triphenylamine (TPyO-TPA), were synthesized through an aldol addition reaction. Their intramolecular charge transfer (ICT) and aggregation emission properties as well as packing structures were investigated. All of them show solvent polarity dependent emission. The density function theory calculations reveal that the ICT characteristic of the HOMO–LUMO transition is responsible for the large solvent effect. In aggregates, PhO-TPA and PyO-TPA exhibit aggregation-induced enhanced emission (AIEE). However, TPyO-TPA displays a different fluorescence behavior. Single-crystal analyses of PhO-TPA and PyO-TPA show that the weak intermolecular interactions and the suppression of the ICT state result in the efficient AIEE. The understanding on the AIEE fluorophores together with ICT characteristic will help to develop materials with color tenability, especially the ones with efficient emission in solid states.

 Please wait while we load your content...
Please wait while we load your content...