The effect of meta versus para substitution on the aggregation of bis-cholesteryl appended 2,6-disubstituted pyridine-based gelators†
Abstract
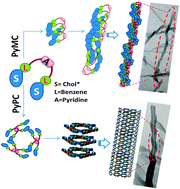
The structurally isomeric, bent core, 2,6-disubstituted pyridine derivatives (PyPC and PyMC) were synthesized, and their gelation ability and aggregation behavior in different solvents were studied using various techniques including electron microscopy (TEM and SEM) and X-ray diffraction analysis (XRD). The PyPC was found to be soluble in most of the organic solvents studied, which may be due to the possible existence of intramolecular hydrogen bonding in PyPC (low angle) that enhanced its solubility. In contrast, PyMC was less soluble, making it a more efficient gelator than PyPC. The results from the morphological studies showed that the xerogel of PyPC in dodecanol exhibited a nanotube morphology (diameter ∼70 nm), whereas PyMC in dodecanol exhibited a fibrous morphology, indicating that the existence of positional isomerism had a profound effect on the aggregation of the molecules in solution. Both right- and left-handed helical structures were observed in all gelators; however, left-handed structures were more predominant. Intermolecular hydrogen bonding as well as π–π stacking cooperatively played an important role in the aggregation of the gelators as evidenced by XRD and temperature-dependent 1H-NMR spectroscopy analyses. Based on these results, aggregation mechanisms were proposed.

 Please wait while we load your content...
Please wait while we load your content...