A sequential one-pot approach to 1,2,4,5-tetrasubstituted-2H-imidazole synthesis from disubstituted alkynes†
Abstract
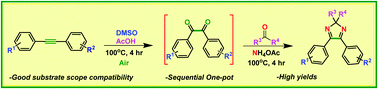
A sequential one-pot approach to the tetrasubstituted 2H-imidazole scaffolds has been developed from disubstituted alkynes and structurally diverse ketones. The reaction proceeds via a diketo intermediate generated from internal alkynes followed by the addition of ammonium acetate and a suitable ketone, affording a diverse range of 2H-imidazoles. Using air-moisture stable reaction conditions and inexpensive reagents, the transformation demonstrates a broad substrate scope and good functional group compatibility.

 Please wait while we load your content...
Please wait while we load your content...