A Pd(ii)/Mg–La mixed oxide catalyst for cyanation of aryl C–H bonds and tandem Suzuki–cyanation reactions†
Abstract
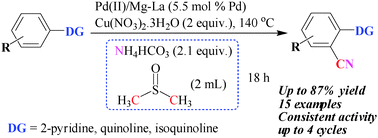
A palladium(II)/magnesium–lanthanum mixed oxide (Pd(II)/Mg–La) catalyst is a reusable catalyst for cyanation of aromatic C–H bonds by using the combination of NH4HCO3 and DMSO as the ‘‘CN’’ source. Moderate to good yields of aromatic nitriles were obtained. An excellent regioselectivity was achieved using the present protocol. A tandem process involving Suzuki coupling followed by a cyanation reaction was also developed using the heterogeneous (Pd(II)/Mg–La) catalyst. This cascade process resulted in the formation of aromatic nitrile from simple 2-halopyridine. The catalyst was recovered by centrifugation and reused for three consecutive cycles with nearly consistent activity and selectivity.

 Please wait while we load your content...
Please wait while we load your content...