Organometallic sulfur complexes: reactivity of the hydrogen sulfide anion with cobaloximes†
Abstract
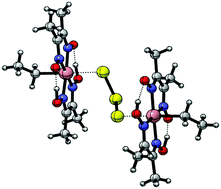
The reaction of alkylcobaloxime [Co(dmgH)2(CH2CH3)(py)] (1) (dmgH = the anion of dimethyl-glyoxime) with KSH in water solution resulted in the selective displacement of the pyridine axial ligand affording the derivative K[Co(dmgH)2(CH2CH3)(SH)] (2). An 1H–1H EXSY experiment revealed that the substitution is reversible and occurs in a slow exchange regime. Attempts to grow crystals of the HS− adduct led to isolation of a rare example of the unexpected trisulfido-bridged dinuclear complex K2[Co2(dmgH)2(CH2CH3)2(μ-S3)]. It was shown that the glyoximato ligands provided the metal centre with a coordination environment quite robust to prevent decomposition and constitute a suitable platform for developing the chemistry of organometallic complexes bearing hydrogensulfido or oligosulfido ligands.

 Please wait while we load your content...
Please wait while we load your content...