Kinetics and equilibrium studies on the removal of aromatic sulfonates from aqueous solution by Mg–Al oxide
Abstract
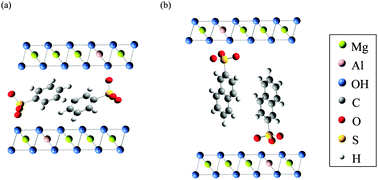
Mg–Al oxide, which was obtained via the thermal decomposition of Mg–Al layered double hydroxides (LDHs) intercalated with CO32− (CO3·Mg–Al LDH), was confirmed to take up benzenesulfonate (BS−) and 2-naphthalenesulfonate (NS−) in aqueous solution. The removal of BS− or NS−, which can be described by pseudo-first-order reaction kinetics, proceeded under chemical reaction control. This process followed a Langmuir-type adsorption; the maximum and equilibrium adsorption constants for NS− were larger than those for BS−. Our results suggests that the Mg–Al oxide reacts more favorably with aromatic sulfonates with lower charge density and that the effect of hydrophobic interactions is larger than that of electrostatic interactions. In the initial reaction stage, Mg–Al oxide rehydrates and combines with an aromatic sulfonate to reconstruct the LDH structure; then, the aromatic sulfonates interact in aqueous solution through hydrophobic interactions, facilitating their removal by the Mg–Al oxide. Because the attraction of NS− molecules is stronger than that of BS− molecules, the removal of NS− by the Mg–Al oxide was found to be more efficient than that of BS−.

 Please wait while we load your content...
Please wait while we load your content...