Reactivity of decafluorobenzophenone and decafluoroazobenzene towards aromatic diamines: a practical entry to donor–acceptor systems†
Abstract
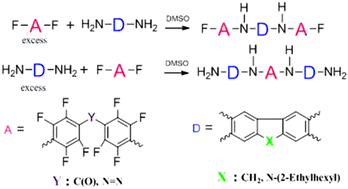
A series of Donor–Acceptor–Donor (D–A–D) and Acceptor–Donor–Acceptor (A–D–A) compounds have been prepared exploiting the relative ability of polyfluorinated azobenzenes and benzophenone to undergo aromatic nucleophilic substitution reactions with aromatic amines. A high para-regioselectivity is obtained when fluorene and carbazole-based diamines have been used in a high Donor Number solvent environment such as DMSO. The prepared triads have been employed in the synthesis of oligomers with the aim of evaluating them as photovoltaic material additives in optoelectronic applications.

 Please wait while we load your content...
Please wait while we load your content...